| |
Schoenfeld MH, Compton SJ, Mead RH, et al. Remote Monitoring of Implantable Cardioverter Defibrillators: A Prospective Analysis. PACE. 2004; 27:757-763.
http://www.blackwellpublishing.com/Blackwell
- SCHOENFELD Hospital of Saint Raphael, Yale University School of Medicine, New Haven, Connecticut,
- STEVEN J. COMPTON - Alaska Heart Institute, Anchorage, Alaska
- R. HARDWIN MEAD - Sequoia Hospital, Redwood City, California
- DANIEL N. WEISS - Florida Arrhythmia Consultants, Delray Beach, Florida
- LOU SHERFESEE - Medtronic, Inc., Minneapolis, Minnesota
- JENNIFER ENGLUND - Medtronic, Inc., Minneapolis, Minnesota
- LUC R. MONGEON - Medtronic, Inc., Minneapolis, Minnesota
- From the *Hospital of Saint Raphael, Yale University School of Medicine, New Haven, Connecticut, †Alaska Heart Institute, Anchorage, Alaska, ‡Sequoia Hospital, Redwood City, California, £Florida Arrhythmia Consultants, Delray Beach, Florida, and §Medtronic, Inc., Minneapolis, Minnesota
Abstract
A prospective study evaluating the functionality and ease of use of the Medtronic CareLink® Network, "CareLink," was conducted at ten investigational sites. This internet-based remote monitoring service allows clinicians to remotely manage their patients' implantable cardioverter defibrillators (ICDs) and chronic diseases. The network is comprised of a patient monitor, a secure server, and clinician and patient websites. Under clinician direction, patients interrogated their ICDs at home, and transmitted data to secure servers via a standard telephone line. Comprehensive device data and a 10-second presenting rhythm electrogram were captured by the monitor and available for access and review on the clinician website. The information could also be printed using a standard desktop computer with internet access. During this study, patients were asked to transmit device data twice, at least 7 days apart, as scheduled by the clinic. Monitor functionality was assessed, and ease of using the system components was evaluated via questionnaires completed by patients and clinicians following each data transmission and review. Fifty-nine patients (64 ± 14 years, range 22-85 years) completed 119 transmissions with only 14 calls to the study support center. Clinician review of data transmissions revealed several clinically significant findings, including silent AF discovery, assessment of antiarrhythmic drug efficacy in a previously diagnosed AF patient, previously unobserved atrial undersensing, and ventricular tachycardia. ICD patients found the monitor easy to use. Clinicians were pleased with the performance of the network and the quality of the web-accessed data, and found it comparable to an in-office device interrogation. CareLink is a practical tool for routine device management and may allow timely identification of clinically important issues. (PACE 2004; 27[Pt. I]:757–763)
Introduction
Medical management of cardiovascular disease has become increasingly reliant upon the use of implanted electronic devices for management of bradycardia, tachycardia, and congestive heart failure.1-3 The simple pacemakers of the 1970s have given way to comprehensive devices, capable of real-time arrhythmia diagnosis and treatment. An implantable cardioverter defibrillator (ICD) patient typically undergoes quarterly device interrogation by radio telemetry, using a dedicated programmer in a device clinic,2 and with the annual U.S. ICD implant rate of 69,000 expected to double, the device follow-up burden is also increasing. 1,4
The growing demands for implantable device follow-up is pushing clinics to their maximum capacity.1,4,5 Any solution that reduces the bottleneck will significantly impact how care is delivered. Remote follow-up, offers an alternative for overcrowded and overwhelmed clinics, and considerable convenience for patients. The use of transtelephonic monitoring (TTM) to remotely assess patients' intrinsic and magnet rates, has helped to reduce the frequency of some clinic visits.6-9 However, TTM usage has been limited to pacemakers, does not provide the complete data available at a clinic visit, and has other limitations including skin electrode placement and potential for telephone noise. Implantable device manufacturers are developing remote follow-up systems that will provide comprehensive data that is easily transmitted by patients and viewed by physicians. Remote interrogation systems that capture automatic device diagnostic data, stored episode electrograms, and the presenting rhythm, provide the clinician with the same information that is available at an office visit used to assess the appropriateness of device therapies and operation. Nearly half of all ICD patients have not experienced tachyarrhythmia episodes at follow-up, or between follow-ups,10 remote interrogations complement and, in some cases replace in-clinic ICD follow-up visits and also serve as a triage tool to determine which patients need further medical attention. Remote follow-up systems, coupled with the tremendous wealth of physiological data collected by the implanted devices, may also be important in the discovery of undocumented and asymptomatic arrhythmias, new disease processes, and the management of chronic diseases including drug initiation and titration. In various clinical studies of remote device monitoring systems, clinicians and patients alike were extremely pleased with the remote follow-up systems with comprehensive device data, and perceived this new technology as an improvement in patient care.6-9 Currently, remote full interrogation functionality is only available for ICD patients, however this functionality had also been identified as a great asset in medical management of the pacemaker population.3
Objectives
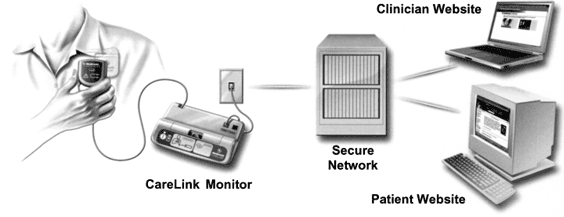
This report focuses on a study of the Medtronic CareLink Network, "CareLink," involving patients with Medtronic GEM II DR ICD (Medtronic, Inc. Minneapolis, MN, USA). The Network, illustrated in Figure 1, includes an FDA-approved portable monitor used by patients to self-interrogate their ICDs, secure servers, and a password protected clinician website where clinicians view and analyze patient device data stored on the server. Patients need a standard analog telephone line to transmit device data; an internet connection is not needed. A patient transmission includes all data within the device memory, including stored episodes, device parameters, and diagnostics. In addition, a 10-second, presenting rhythm electrogram is captured at the time of the interrogation. The device data captured are comparable to an in-office interrogation and can be conveniently reviewed on the clinicians' computer or printed in a format similar to that obtained from an in-office programmer. The patients have the option to use a personalized, password protected patient website to view a high level summary of their device information as well as educational information about living with an ICD.
The specific objectives of the Medtronic CareLink Network ICD study were to: (1) evaluate the ease of use of the monitor by the user (patient, family member, or assistant) in their home; (2) evaluate patient satisfaction with the monitor; (3) evaluate clinician satisfaction with reviewing device data via the clinician website; and (4) assess the level of complexity for troubleshooting calls to the support center. In addition, clinical observations related to device/programming concerns and disease management issues were compiled.
The Medtronic CareLink® Network.
Design/Methods
The CareLink evaluation used a prospective, multicenter, nonblinded, nonrandomized study design. Patients included in the study had a Medtronic GEM II DR (Medtronic Inc.) device and signed a consent form. The Institutional Review Board of each participating center approved the study protocol. Only patients that did not have access to a standard telephone line were excluded from this investigation. Patients received study training and an overview of the monitor operation before their first transmission.
Clinicians used the on-line enrollment feature to enroll patients. The monitor was then shipped directly to the patients' homes, along with an instructional package that included a training video, a patient manual, and a quick reference card.
Upon receiving their monitors, patients transmitted their device data per clinic instructions. Patients were asked to send two transmissions with a minimum of 7 days between transmissions. Patients were required to contact the support center after each transmission to provide feedback on using the monitor. "Troubleshooting" phone calls were noted when the patients were unable to complete their data transmission. The complexity of each troubleshooting call was evaluated by whether the support center representative triaged the phone call to a technical expert. All troubleshooting calls were analyzed.
Descriptive statistics were used to summarize patient satisfaction with the monitor. The percentage (mean ± standard deviation) of successful transmissions that did not require a troubleshooting phone call was calculated for both the first and second transmissions. For those patients who completed two transmissions and two questionnaires, a pooled percentage was computed. Patients' assessment of the ease of monitor setup and usefulness of the reference materials was summarized.
All remote interrogations were reviewed by the enrolling clinics. Any device related or patient/disease related observations were compiled. Each clinic interpreted the data and took clinical actions per their standard of care. Clinicians were asked to complete a written questionnaire after reviewing each transmission.
Clinic and Patient Population Description and Characteristics
Fifty-nine Medtronic GEM II DR (Medtronic, Inc.) patients, age 64 ± 14 years (range: 22–85 years), participated in the study at 10 follow-up clinics. Forty-five (76.3%) of the patients were male. Forty patients (68%) had history of ventricular tachycardia, and 15 patients (25%) had a history of sudden death. Twenty-six patients (44%) were in New York Heart Association (NYHA) Class I, 20 (34%) were Class II, and 10 (17%) were Class III patients. NYHA class was unavailable for three patients. The average distance the study patients needed to travel to their clinics was 41 miles (range 1–300 miles). The investigational clinics were geographically diverse. Fifty-seven (97%) patients lived independently while two (3%) of the patients needed assistance with daily living.
Ease of Use of the Patient Monitor
Fifty-three patients completed two transmissions at least 7 days apart along with corresponding questionnaires. Two additional patients completed two transmissions, but valid questionnaires were only available for one transmission per patient. In total, 110 transmissions from 55 patients were used in the monitor ease of use analysis, while 106 patient questionnaires from 53 patients were used for the patient satisfaction analysis. Patients did not require outside assistance in 91% of the 110 transmissions used in the pooled analysis. Ninety-three percent of second transmissions were assistance free. Of those patients with assistance free transmissions, 71% used all reference materials during the first transmission and 54% used all reference materials for the second transmission. Six patients successfully completed the second transmission without using any materials.
Following a successful transmission, patients rated the ease of setup and antenna positioning. The results are summarized in Table I. More than 98% of the pooled responses indicated that the monitor was very easy, or somewhat easy to setup. One hundred percent of respondents rated the monitor setup as very or somewhat easy after their second transmission. In 86% of the responses, the patient found the antenna very easy, or somewhat easy to position. One patient rated the ease of positioning the antenna as very difficult after both transmissions. Two other patients rated it as somewhat difficult after both transmissions.
Patient Satisfaction with the Monitor
There were 106 responses from 53 users available for comparative analysis of patient satisfaction with the monitor. One hundred four (98.1%) of these responses indicated that the monitor was either somewhat easy to use or very easy to use.
Patient satisfaction by transmission is presented in Table I. The pooled results include responses from users that completed both transmissions and corresponding questionnaires. A family member provided a response in 15 of the 106 cases, while patients responded in the remaining cases. One patient, who rated the monitor as somewhat difficult to use, required assistance with antenna placement and transmitting from work, which required moving a switch on the monitor to dial an outside number. Another patient mistook the antenna positioning light for the battery light, and changed the batteries prior to a second transmission. A third patient had difficulty positioning the antenna during a second transmission, and rated the device as very difficult to use.
Patient Feedback on the Medtronic CareLink Monitor.
|
Very
Easy |
Somewhat
Easy |
Somewhat
Difficult |
Very
Difficult |
Total
|
Ease of Set UP |
First Transmission |
52 (89.7%) |
4 (6.9%) |
2 (3.4%) |
0 (0.0%) |
58 (100%) |
Second Transmission |
50 (94.3%) |
3 (5.7%) |
0 (0.0%) |
0 (0.0%) |
53 (100%) |
Pooled Results |
102 (91.8%) |
7 (6.3%) |
2 (1.8%) |
0 (0.0%) |
111 (100%) |
Ease of Antenna Positioning |
First Transmission |
36 (62.1%) |
15 (25.9%) |
6 (10.3%) |
1 (1.7%) |
58 (100%) |
Second Transmission |
31 (58.5%) |
14 (26.4%) |
7 (13.2%) |
1 (1.9%) |
53 (100%) |
Pooled Results |
67 (60.3%) |
29 (26.1) |
13 (11.7%) |
2 (1.8%) |
111 (100%) |
Patient Satisfaction |
First Transmission |
53 (91.4%) |
4 (6.9%) |
1 (1.7%) |
0 (0.0%) |
58 (100%) |
Second Transmission |
49 (90.7%) |
3 (5.6%) |
1 (1.85%) |
1 (1.85%) |
54 (100%) |
Pooled Results** |
97 (91.5%) |
7 (6.6%) |
1 (0.95%) |
1 (0.95%) |
106 (100%) |
*One patient completed two patient questionnaires for his first transmission. These data were omitted. |
**Excludes patients who did not complete two transmissions and patient questionnaires |
Clinician Satisfaction with Reviewing Device Data Remotely
Clinicians completed a clinician questionnaire following the review of each patient's device data. One hundred eleven (96.5%) of the 115 responses indicated that the clinician was either somewhat or very satisfied with the clinician website for viewing device data. Table II shows clinician satisfaction by clinic role, viewing location, and internet access. All clinicians viewed the website with Microsoft Internet Explorer version 4.0 or higher. Amount of time needed to access the patient screen was evaluated. A total of 114 (98.3%) of the 116 clinician responses indicated that the amount of time needed to access the patient data was excellent or very good. The majority (96.5%) of responses indicated the clinician found navigating the website very or somewhat easy. Four responses (all from one clinician) indicated that the website was somewhat difficult to navigate. Eighty-eight percent and 94% of clinician responses rated the ease of reviewing patient data on the screen or in printed form, respectively, as easy to read. After reviewing patient data on the website, clinicians indicated 96.5% of the time that the remote monitoring service allowed them to provide care comparable to an in-office, interrogation-only visit.
Troubleshooting Calls
All 119 transmissions completed during the study were included in this analysis whether or not they were protocol compliant, since these data provide valuable feedback on the CareLink monitor. Each time a patient called the study support center for assistance, the troubleshooting section of the patient questionnaire was completed. Five out of 14 troubleshooting calls were triaged for additional technical assistance. Four of the calls regarded problems with antenna positioning, and one required that the patient change a switch on the monitor to dial 9 for an outside line. Two patients needed additional training, and one patient needed reminding that the transmission phone number is preprogrammed in the monitor. Patients used the instructional materials prior to making troubleshooting calls in 8 (57.1%) of 14 cases. Ten (71.4%) of the 14 calls involved problems with antenna positioning. In all cases, patients were able to transmit following the troubleshooting call.
Clinician Satisfaction with the Medtronic CareLink Clinician Website.
|
N |
Number (Percentage) of Responses |
Very or Somewhat
Satisfied |
Somewhat
Dissatisfied |
Very
Dissatisfied |
Position |
Physician |
20 |
20 (100.0%) |
0 (0.0%) |
0 (0.0%) |
Nurse |
90 |
86 (95.6%) |
4 (4.4%) |
0 (0.0%) |
Other |
5 |
5 (100.0%) |
0 (0.0%) |
0 (0.0%) |
Viewing Location |
Office |
112 |
108 (96.4%) |
4 (3.6%) |
0 (0.0%) |
Home |
3 |
3 (100.0%) |
0 (0.0%) |
0 (0.0%) |
Internet Access |
Network/DSL/Cable |
65 |
61 (93.8%) |
4 (6.8%) |
0 (0.0%) |
Phone/Modem/Dial-up |
50 |
50 (100.0%) |
0 (0.0%) |
0 (0.0%) |
All Clinicians |
115* |
111 (96.5%) |
4 (3.5%) |
0 (0.0%) |
*One clinician questionnaire was missing a score for overall satisfaction with the website |
Note that each time a clinician viewed a patient transmission, he/she indicated a response for that particular transmission review. For those clinicians rating different internet access, some indicated dissatisfaction at the speed and accessibility to the internet via the network in their office. |
Clinical Observations
Table III describes the clinical observations noted after thorough review of the transmitted device data. The observations are classified as device or patient related. All device related observations were made from the 10-second presenting rhythm electrogram captured at the time of interrogation.
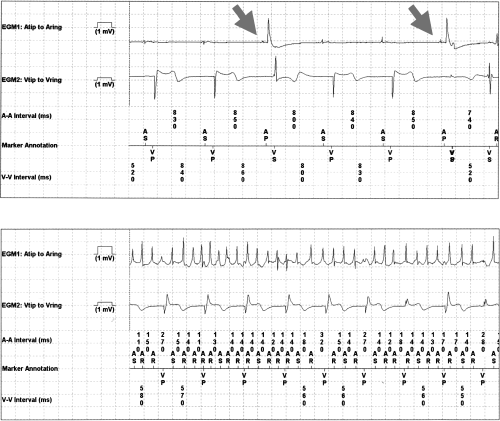
One patient with significant atrial undersensing (Fig. 2, Top) was subsequently brought into the clinic and reprogrammed to remedy the situation. In another patient, T wave and far-field R wave sensing were noted, but it did not lead to inappropriate therapy delivery.
Nonsustained ventricular tachycardia (VT) was seen in one patient. In another patient, an episode was classified as VT by the ICD and treated, but after careful analysis, was determined to be an SVT with Wenckebach conduction. In a third patient, new onset atrial fibrillation was discovered during the remote interrogation, and the physician initiated anticoagulation therapy (Fig. 2, Bottom).
Device and Patient Related Observations.
Device Related Observation
(10 second presenting rhythm EGM) |
Number of Patients |
Notes
|
Atrial undersensing |
2 |
Intermittent, one patient brought back monitor and reprogrammed |
T wave oversensing |
2 |
Did not lead to inappropriate detection or therapy delivery |
Far-field R wave sensing |
4 |
Intermittent in all patients. Did not lead to inappropriate therapy delivery |
Safety Pace |
3 |
Proper device operation |
Patient Related Observation |
Atrial Fibrillation: |
10 second interrogation EGM |
5 |
One patient with new AF discovery, started anticoagulation therapy |
Mode Switches (device log) |
15 |
|
VT Episodes (stored episodes) |
2 |
One nonsustained |
|
|
One treated slow VT |
Ventricular Ectopy |
14 |
Captured by the 10 second interrogation EGM |
Large Changes in% pacing (device log) |
6 |
Potential indicator of changing patient condition or poor device operation especially with respect to sensing and pacing. |
EGM = electrogram from the implantable defibrillator; VT = ventricular tachycardia; AF = Atrial fibrillation |
Device and patient related events discovered on the 10-second presenting electrogram.
Top: Atrial undersensing was discovered using the 10-second electrogram captured at interrogation, and the patient was prerecorded.
Bottom: AF discovery was seen during the 10-second electrogram captured at interrogation. Anticoagulation therapy was initiated.
AS = atrial sense; AP = atrial pace; VS = ventricular sense; VP = ventricular pace.
Discussion
Independent evaluations of remote monitoring systems indicate that ICD patients are capable of using this new technology to interrogate their implanted devices, and felt that the quality of their care improved.7 Patients currently using the CareLink system have described a sense of reassurance created by the remote monitoring capability. In particular, the appropriateness of device discharges and the need for immediate/emergent versus delayed follow-up may be addressed.11 Clinicians using CareLink to manage their device patients have reported several instances where emergency room visits were averted, new-onset atrial fibrillation was discovered, and monitoring of antiarrhythmic medication effects after a dosage change was conducted. Clinicians indicate that comprehensive remote monitoring is an easy and effective way to examine their patients' ICD information. In addition, clinicians are able to identify potential programming irregularities, identify lead issues, diagnose previously unknown arrhythmias, and optimize device parameters.
The aging population, the proliferation of automatic external defibrillators, and expanded indications for device use is expected to dramatically impact device clinics.12-15 Approaches and methods that improve clinic efficiency are necessary to maintain today's standard of care. For those ICD patients who come into the office for routine device checks with few or no episodes, the clinician may choose to have the majority of follow-ups done remotely, and only bring patients who need further assistance into the office following review of their transmitted data. Acute events like symptoms or shocks that once required an unscheduled office or emergency room visit can now be remotely reviewed, and a clinical decision made quickly. Frequent device monitoring is needed immediately following an implant and when the device approaches end-of-life.16 Remote monitoring can make these time periods more efficient and convenient for both the clinician and patient.
Technologies like CareLink offer the promise of improved clinical efficiency and disease management from home or while traveling, and will impact the way medical care is delivered to patients with implantable cardiac devices. However, to achieve the remote disease management vision, these initial claims must be proven before remote monitoring can become the standard of care for following patients with implanted cardiac devices. Such technology cannot provide information provided by direct physical examination or radiography, and therefore may not totally supplant in-clinic outpatient visits, but may certainly facilitate follow-up.16
Conclusions
A representative population of ICD patients effectively used the CareLink, and patient satisfaction with the monitor was very high. Clinicians were also satisfied with CareLink, the accessibility to patient data, and the ability to provide patient care comparable to an in-office, interrogation-only visit. Remote monitoring has implications on the clinical practice of device follow-up, as well as disease and drug management, as illustrated in this evaluation. While this protocol did not allow for patient–initiated transmissions on the basis of symptoms such as perceived device discharges, an anticipated advantage of CareLink would be to evaluate such transmissions to determine whether a shock was appropriate or spurious, thus minimizing patient emergency room and office visits. Additional assessment of this technology's impact on disease management, patient outcomes, quality-of-life, and economic endpoints will further demonstrate the usability and clinical value of remote monitoring for patients with implantable cardiac devices.
References
1. Winters SL, Packer DL, Marchlinski FE, et al. Consensus statement on indications, guidelines for use, and recommendations for follow-up of implantable cardioverter defibrillators. PACE 2001; 24: 262–269.
2. Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation 2002;106: 2145–2161.
3. Bernstein AD, Irwin ME, Parsonnet V, et al. Report of the NASPE Policy Conference on antibradycardia pacemaker follow-up: Effectiveness, needs, and resources. North American Society of Pacing and Electrophysiology. PACE 1994; 17: 1714–1729.
4. McGrory-Usset ME, Stanton MS. Use of Implantable Cardioverter Defibrillator (ICD) Therapy and ICD Guidelines Around the World. In IE Ovyshcher (ed.): New Development in Cardiac Pacing and Electrophysiology. Armonk, NY: Futura Publishing Company Inc., 2002, pp. 81–90.
5. Frye WB. Cardiology workforce: There's already a shortage, and it's getting worse! J Am Coll Cardiol 2002; 39: 2077–2079.
6. Schoenfeld MH. Follow-up of the Paced Patient. In KA Ellenbogen et al. (eds.): Clinical Cardiac Pacing and Defibrillation, 2nd edition. Philadelphia, PA: WB Saunders Company, 2000, pp. 895–929.
7. Dressing TJ, Schott R, McDowell C, et al. Transtelephonic ICD Follow-up is Better: More Comprehensive, Less Intrusive and More Desirable. (abstract) PACE 2002; 25: 219.
8. Igidbashian D, Stellbrink C, Hartmann A, et al. Benefit of permanent pacemaker follow-up with home monitoring. PACE 2002; 25: 47.
9. Stellbrink C, Hartmann, A, Igidbashian D, et al. Home monitoring for pacemaker therapy: Intermediate results of first European multicenter study. PACE 2002; 25: 655.
10. Medtronic Inc. Industry Data on file. 2001.
11. Owen, R. Pacer/arrhythmia clinic improves patient satisfaction. EP Lab Digest 2003; 3: 18–19.
12. The AVID Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators. N Engl Med 1997; 337: 1576–1583.
13. Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. . N Engl Med 1999; 341: 1882–1890.
14. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl Med 1996; 335: 1933–1940.
15. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl Med 2002; 346: 877–883.
16. Schoenfeld MH, Markowitz HT. Device follow-up in the age of automaticity. (editorial) PACE 2000; 23: 803–806.
This article has been reprinted with permission via Rightslink.
Brief Statement
Intended Use: The Medtronic CareLink® Monitor is indicated for use in the transfer of patient data from some Medtronic implantable cardiac devices based on physician instructions and as described in the product manual. This product is not a substitute for appropriate medical attention in the event of an emergency and should only be used as directed by a physician.
Contraindications: There are no contraindications for the Medtronic CareLink Monitor.
Warnings and Precautions: The Medtronic CareLink Monitor must only be used for interrogating compatible Medtronic implantable devices. Do not use a cellular phone while the antenna is positioned over the implanted device.
See the device manual for detailed information regarding the instructions for use, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1 (800) 328-2518 and/or consult Medtronic’s website at www.medtronic.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. |


